Case Study
Biocept sets sights on expansion, fueled by COVID-19 testing
ServiceRegulatory/Permitting Support
ServiceStrategic Partnerships
Biocept develops and performs molecular diagnostic assays that provide physicians with clinically actionable information for treating and monitoring patients. Fueled by its pivot to meet COVID-19 testing demands, Biocept needed to relocate to support its expansion with minimal interruption to services.
Company
Challenge
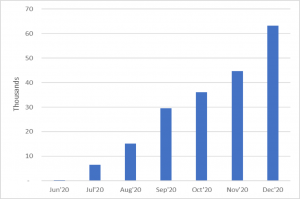
San Diego-based Biocept began offering PCR-based COVID-19 testing services in June 2020, when the company determined that its expertise in molecular testing for cancer could be leveraged to assist during the pandemic. The effort has been a major success—Biocept has currently received more than 380,000 samples since its launch. With its current lease expiring, the company was looking for a new site that could better suit its needs to handle the growing demand for its testing services, as sample processing capacity ramped from 2,500 to 5,000 tests per day.
During the relocation, Biocept needed to minimize delays to its ongoing COVID-19 and cancer sample processing, and required strategic planning support to safely transfer its machinery and equipment to the new site.
EDC Solution and Support Provided
From mid-August through December 2020, EDC connected Biocept with key permitting and planning resources in preparation for its move.
In doing so, EDC supported Biocept’s move and expansion within San Diego’s Sorrento Valley biotech hub with minimal interruption for patients with cancer and for those who needed COVID-19 testing.

Value Derived
Biocept has grown rapidly, increasing its full-time employee count from 93 to 112 to support its expanded processing capacity at the new location. Biocept also hired 30 interns and 28 temporary employees.
During this time, the company successfully ramped up its test sample processing capacity from several thousand to more than 60,000 samples processed per month. Biocept also accelerated the commercial launch of its new CNSide cerebrospinal fluid assay offering for cancers that have metastasized to the brain. The move reduced overhead expenses, was completed with minimal impact to patients, customers, and employees, and now saves the company approximately 20 percent annually in facility-related expenses.
“At Biocept, we are always looking for ways to grow so that we can better meet the needs of physicians and their patients. Thanks to EDC’s permitting support and partnership, as well as their colleagues in the city planning department, we were able to expand our operations within the Sorrento Valley innovation hub, where we have been able to take advantage of San Diego’s top technical talent.”
—Michael Nall, President and CEO, Biocept
Connect with Biocept
- Website: https://biocept.com/
- Twitter: https://twitter.com/Biocept
- LinkedIn: https://www.linkedin.com/company/biocept/
WANT TO KNOW MORE?
Check out our LIFE SCIENCES industry profile
See HOW WE CAN HELP YOUR COMPANY
