 From 2025 to 2050, the 65-and-older population is projected to almost double to 1.6 billion globally, whereas the total population will grow by just 34 percent over the same period. With this, it has become increasingly important to support our aging population, with health and wellness among top priority.
From 2025 to 2050, the 65-and-older population is projected to almost double to 1.6 billion globally, whereas the total population will grow by just 34 percent over the same period. With this, it has become increasingly important to support our aging population, with health and wellness among top priority.
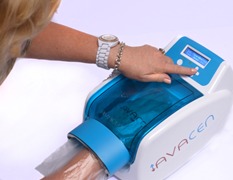
San Diego medical technology company and 2016 MetroConnect participant AVACEN Medical has developed technology to help ease some of the common ailments afflicting seniors. The AVACEN 100 is an FDA cleared, over-the-counter medical device that provides non-invasive, temporary arthritis and muscle pain relief, and muscle relaxation. Using microcirculation enhancement on the palms, the locally-made device helps warm and thin the blood, thereby dissipating heat throughout deep tissues and relieving joint pain associated by arthritis, muscle spasms, sprains and more.
Taking this San Diego-made technology global, the AVACEN 100 has just received the CE (Conformité Européenne) Mark approval to treat widespread pain associated with fibromyalgia. The CE Mark allows AVACEN to market its AVACEN 100 to the European Union’s 28 member countries where many prescription drugs, available in the U.S., have been rejected by regulatory officials for treating fibromyalgia pain.
Founded by Tom Muehlbauer in 2009, AVACEN’s revolutionary technology was originally developed to help alleviate his sister-in-law’s chronic pain. The company currently sells in two countries, with plans to expand into 10 more over the next year (thanks in part to the CE Mark). Sales have climbed to more than $1.5 million, with more than 20 percent of the sales coming from international markets.